Neuralink Launches Patient Registry to Enhance Brain Implant Technology
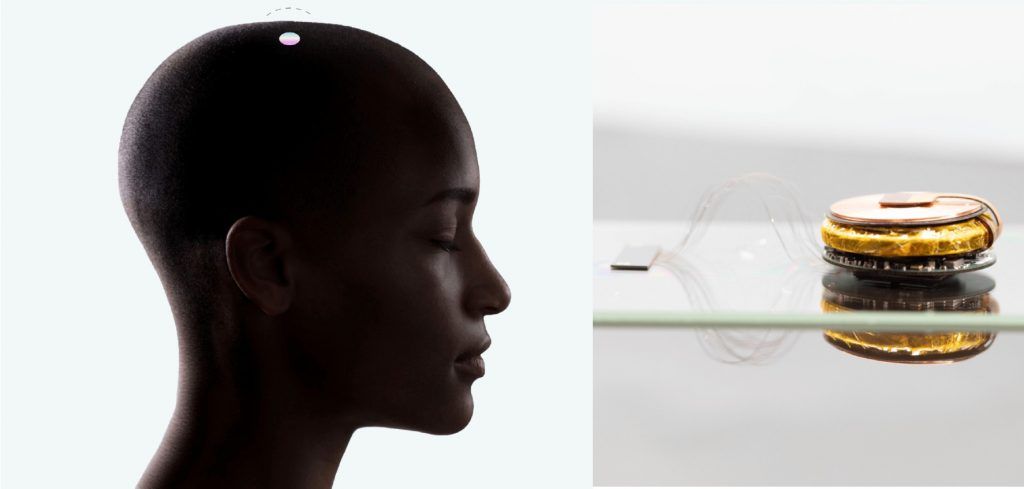
Neuralink, the brain-machine interface (BMI) developer founded by Elon Musk, has recently launched a patient registry aimed at increasing the company's understanding of the medical and assistive technology needs of individuals with certain medical conditions. The registry is expected to help Neuralink design future clinical trials and neurotechnology devices that meet these needs.
The conditions that Neuralink is focusing on in its patient registry are tetraplegia or tetraparesis, paraplegia, vision loss, deafness, and aphasia. Tetraplegia is a condition where patients are paralyzed or unable to move all their limbs, while paraplegia only affects two limbs. Vision loss and deafness are self-explanatory, while aphasia is a language disorder that results from damage to the brain, causing a person to be unable to communicate effectively.
The goal of Neuralink's BMI technology is to allow people with these conditions to control computers, phones, and other devices with their minds. The technology could also enable people to control prosthetic limbs or even restore lost functions, such as eyesight and hearing.
To participate in the patient registry, individuals must be at least 18 years old and either a US citizen or a permanent resident. While participants will not be compensated for their involvement in the registry, those who meet the criteria for Neuralink's human clinical trials may be contacted again by the company for more information and enrollment. However, the company has emphasized that enrollment in the patient registry does not guarantee eligibility for clinical trials.
During the "Show and Tell Fall 2022" event, Elon Musk stated that Neuralink could receive approval for human clinical trials in the first half of 2023. The company has already submitted paperwork to the US Food and Drug Administration (FDA) seeking approval for its clinical trials.
Neuralink faces competition in the BMI field from developers like Synchron, which has already started human clinical trials in Australia and the United States. Synchron's human trials have shown promising results thus far.